How does experience change the brain?
Structural Brain Plasticity & Neurodegeneration
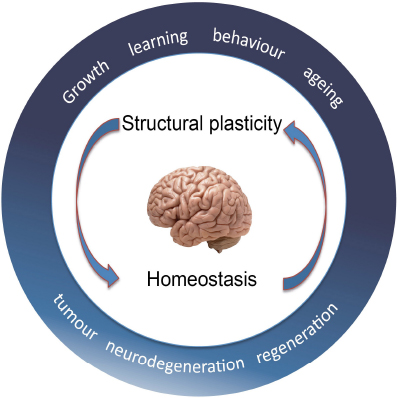
Why do we learn better when young? Why do we forget when old? Why is sport good for the brain? Why is isolation bad? We want to understand how structural changes in cells determine how the brain works. As we grow, the number of neurons and glia increases as the brain expands and neural circuits are laid down, neurons establish connectivity patterns between dendrites and axons and form synapses. As we go through life, as we learn and age, cells can change, resulting in modifications in cell number, dendrites, axons and synapses. Structural brain plasticity may enable adaptation, distribute memory and modify behaviour. Plasticity is counteracted by structural homeostasis, which constrains change, and together they enable appropriate behaviour. When the balance between plasticity and homeostasis breaks down, it can result in neurodegeneration, psychiatric disorders and brain tumours. Conversely, by understanding the mechanisms balancing structural plasticity and homeostasis, we might be able to direct regeneration and repair upon central nervous system injury and disease.
To address these questions, we use the fruit-fly Drosophila as a model organism, for its powerful genetics and the opportunities it offers to manipulate genes and visualise neurons, glia, neural circuits and behaviour. Findings from Drosophila expedite research, uncovers fundamental principles on how any brain works and contribute to the understanding also of the human brain, in health and disease.
Glia, Regeneration & Repair
in the central nervous system
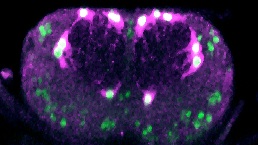
How do cells know what makes a functional nervous system? and how do they know how to interact with other cells types – neurons vs. glia – to achieve this? They know so during development, so do they retain this ability throughout life? and if they don’t, can we re-awaken it to promote regeneration and repair? The human central nervous system (brain and spinal cord) does not regenerate upon injury, damage or disease. However, some animals can regenerate the CNS. If we could understand how cells organise themselves into functional neural circuits in development and in regenerating animals, we might be able to direct cells to promote regeneration and repair after injury or disease. Using the fruit-fly Drosophila, we have discovered a gene network that promotes glial regeneration after injury. Glial cells clear cell debris resulting from the injury, close the lesion and restore their own cell populations, required for normal neuronal function. We have shown that eliminating genes of the discovered gene network or having them work in excess, we can change the response to injury from prevention to promotion of repair. We have recently found that upon injury and under certain conditions, glial cells can also acquire neurogenic potential. In vivo reprogramming of glial cells unto neural stem cells that could produce neurons, would have tremendous potential for regenerative medicine.
The Pathogen & the Brain
When a pathogenic bacteria or fungus approaches us, we produce antimicrobial peptides to combat infection, so that we can continue to stay healthy and thrive. How does the pathogen perceive their interaction with us? Do pathogens combat our defences so that they can survive, or further, are we a good meal to them?
Fungi evolve within the host, ensuring their own nutrition and reproduction, at the expense of host health. They intervene in hosts’ brain function, to alter host behaviour and induce neurodegeneration. In humans, fungal infections are emerging as drivers of neuroinflammation, neurodegenerative diseases and psychiatric disorders. However, how fungi alter the host brain is unknown. Fungi trigger an innate immune response mediated by the Toll-1/TLR receptors, the adaptor MyD88 and the transcription factors Dif/NFκB, that induce the expression of antimicrobial peptides (AMPs). However, in the nervous system, Toll-1/TLR could also drive an alternative pathway involving the adaptor Sarm, which causes cell death instead. Sarm is the universal inhibitor of MyD88 and could drive immune evasion. The entomopathogenic fungus Beauveria bassiana is well-known to activate Toll-1 signalling in innate immunity in Drosophila. Furthermore, in fruit-flies, the adaptor Wek links Toll-1 to Sarm. Thus, here we asked whether B. bassiana could damage the Drosophila brain via Toll-1, Wek and Sarm. Our data show that B. bassiana causes cell loss in the host brain via Toll-1/Wek/Sarm signalling driving immune evasion. We conclude that pathogens can benefit from an innate immunity receptor to damage the host brain. A similar activation of Sarm downstream of TLRs in response to fungal infections could underlie psychiatric and neurodegenerative diseases in humans.
Imaging
In order to address questions into structural plasticity, we generate software programmes that enable us to automatically count neurons, glia, dying cells, dividing cells, synapses, throughout large stacks of confocal microscopy images that cover the entire central nervous system of the fruit-fly Drosophila. This is fast and accurate. This allows us to use large samples sizes and compare multiple genotypes in each experiment. The programmes are all written as ImageJ plugins and are publicly available through this website.